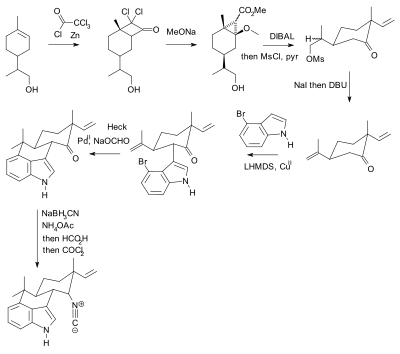
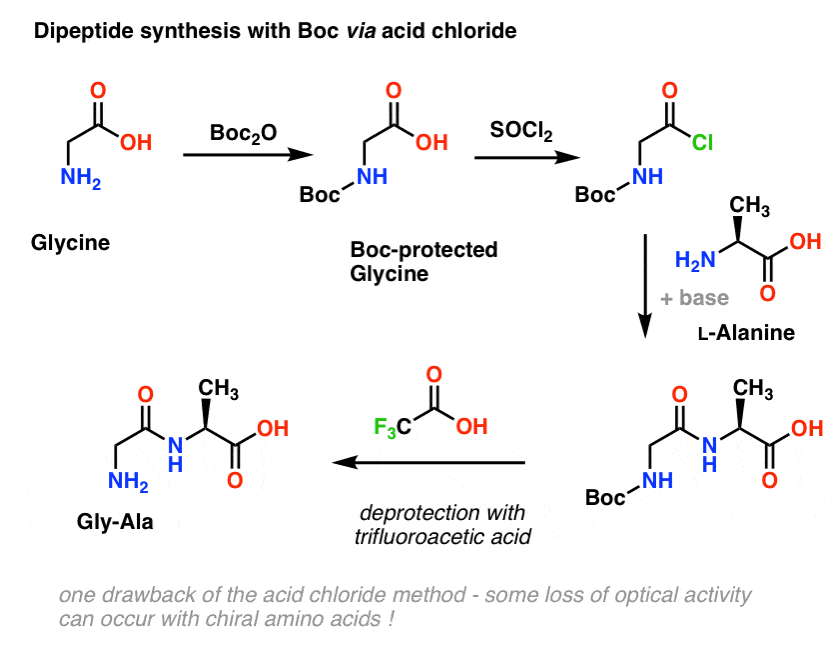
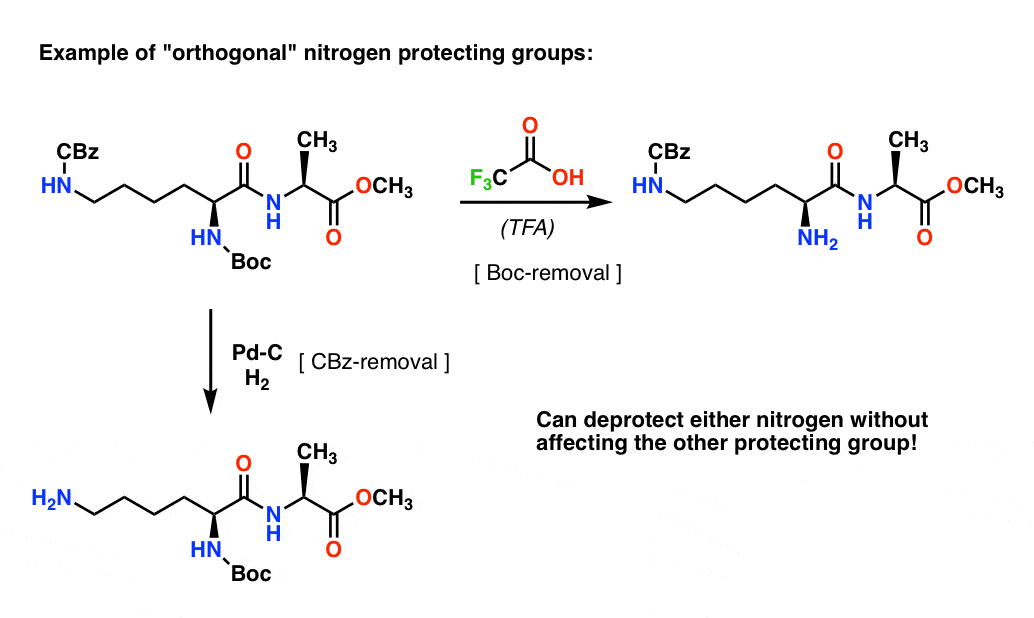
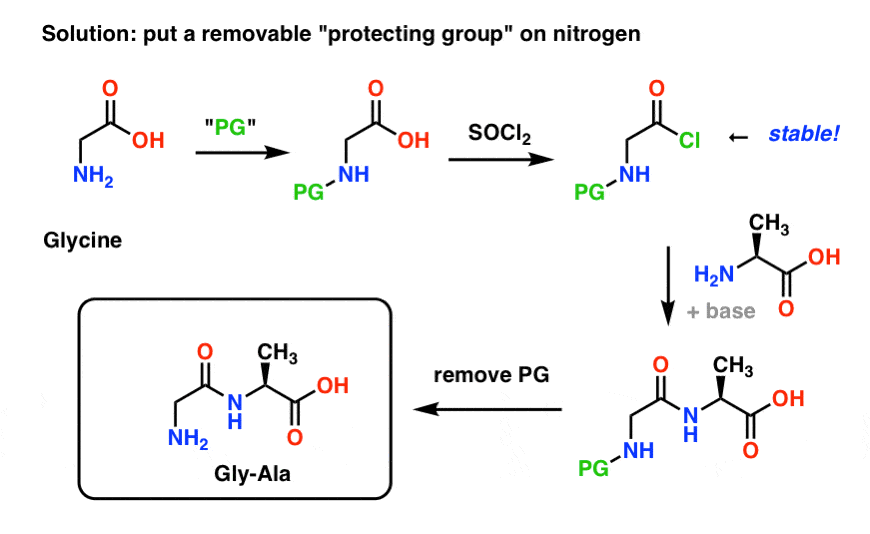
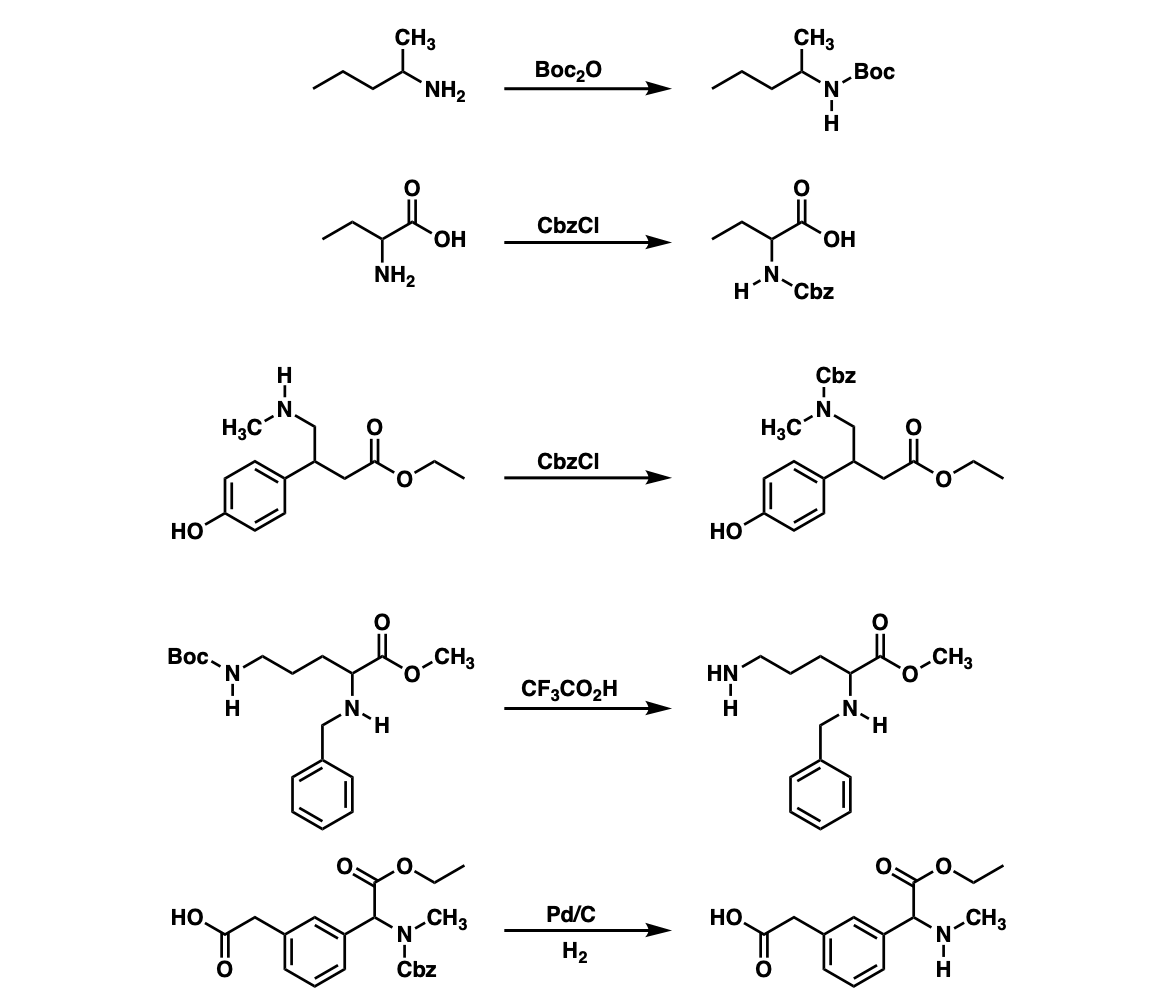
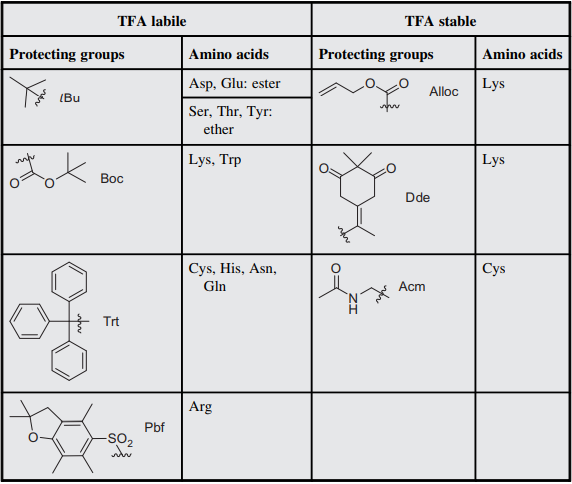
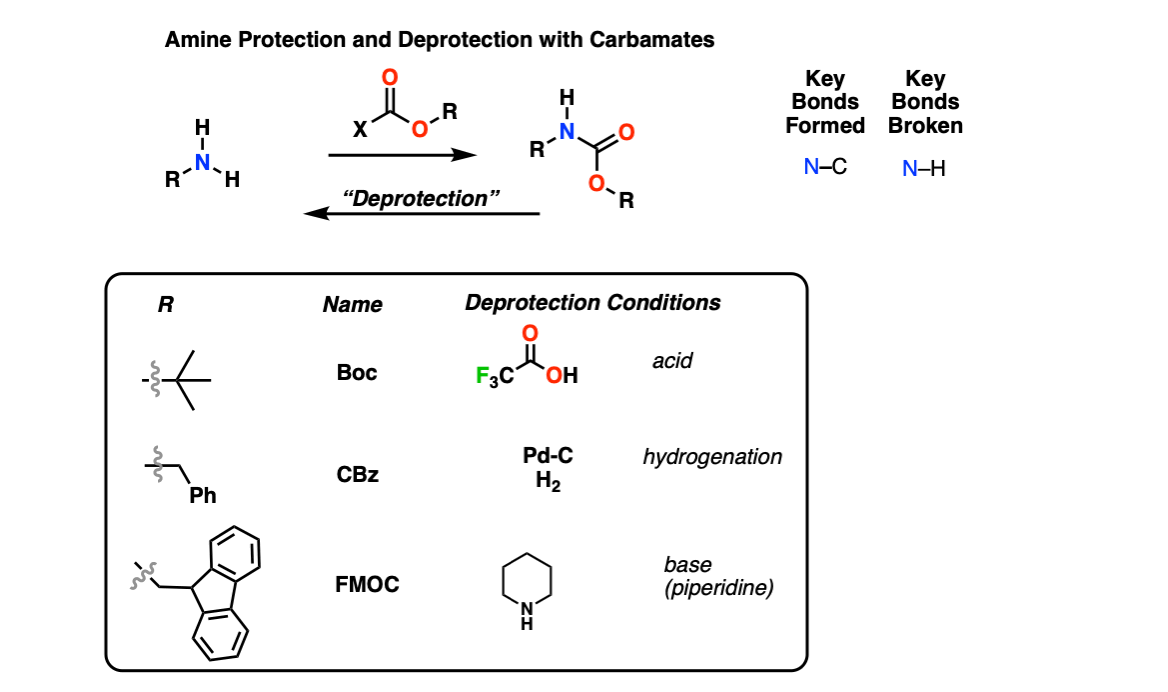
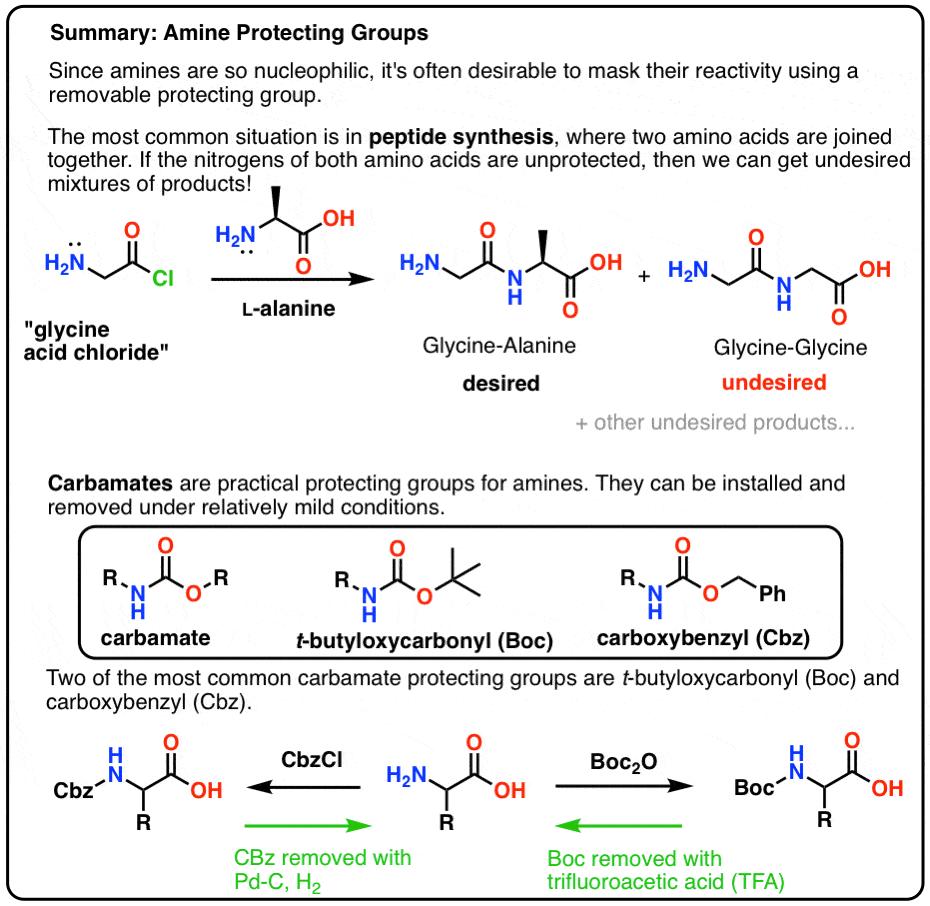
Selective Protection and Deprotection of Primary Amino Group Using 1,3-Dimethyl-5-acetylbarbituric Acid | TCI AMERICA

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications

Synthesis of Sulfonimidamide‐Based Amino Acid Building Blocks with Orthogonal Protecting Groups - Chinthakindi - 2019 - European Journal of Organic Chemistry - Wiley Online Library

Unlocking the Potential of Phenacyl Protecting Groups: CO2-Based Formation and Photocatalytic Release of Caged Amines | The Journal of Organic Chemistry